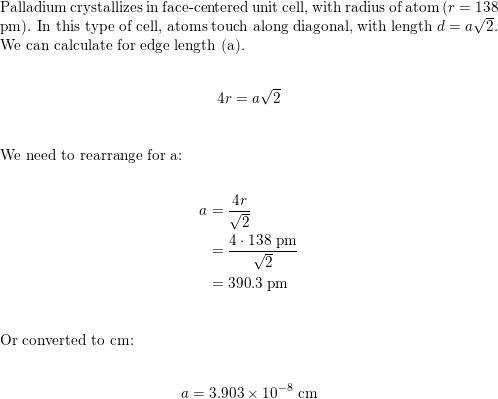OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

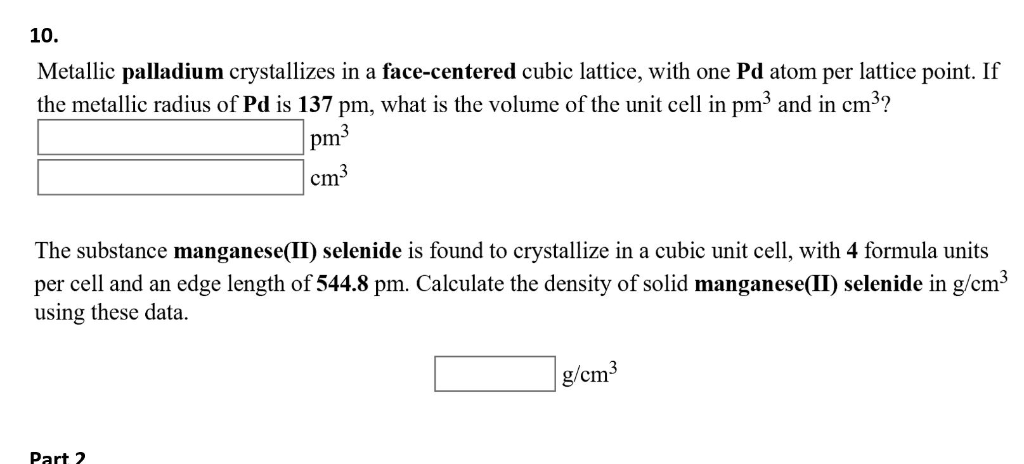
Metallic lead crystallizes in a face-centred cubic lattice, with one Pb atom per lattice point. If the metallic radius of Pb is 175 pm, what is the volume of the unit cell
PLEASE HELP! 80 points!! A metal crystallizes in the face‑centered cubic (FCC) lattice. The density of the - Brainly.com
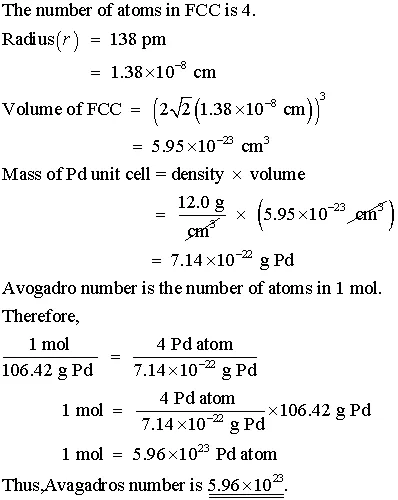
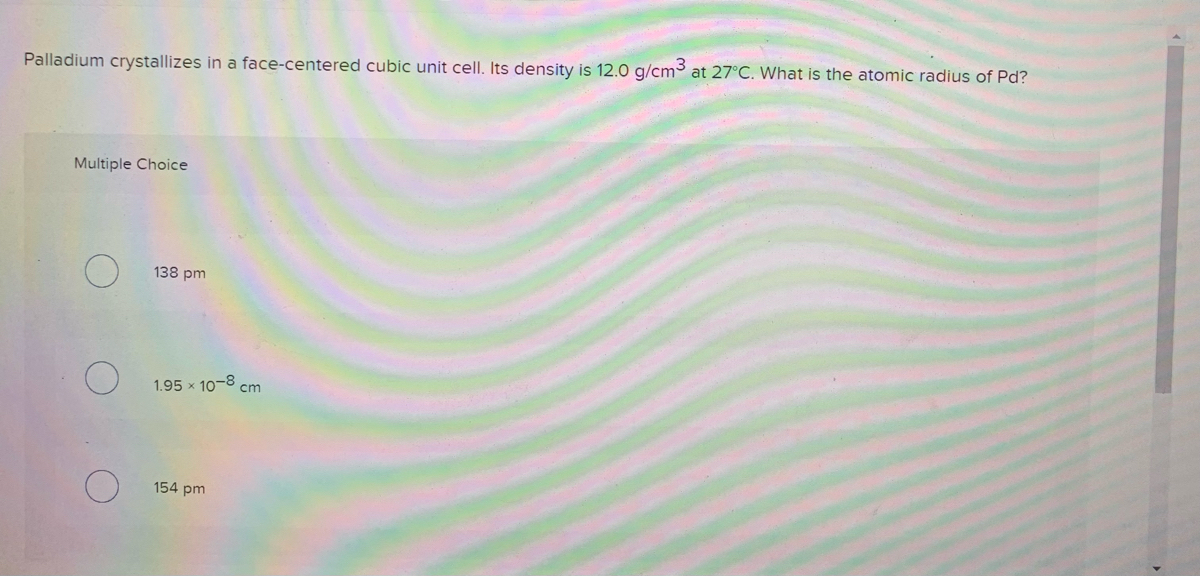
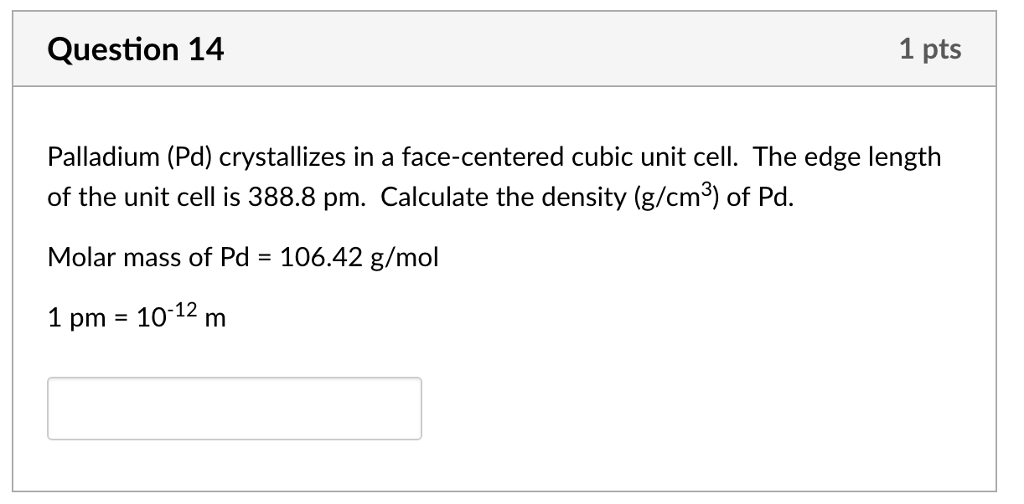
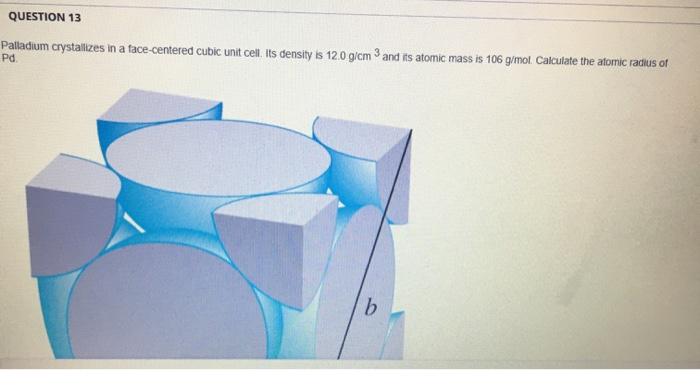
OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...
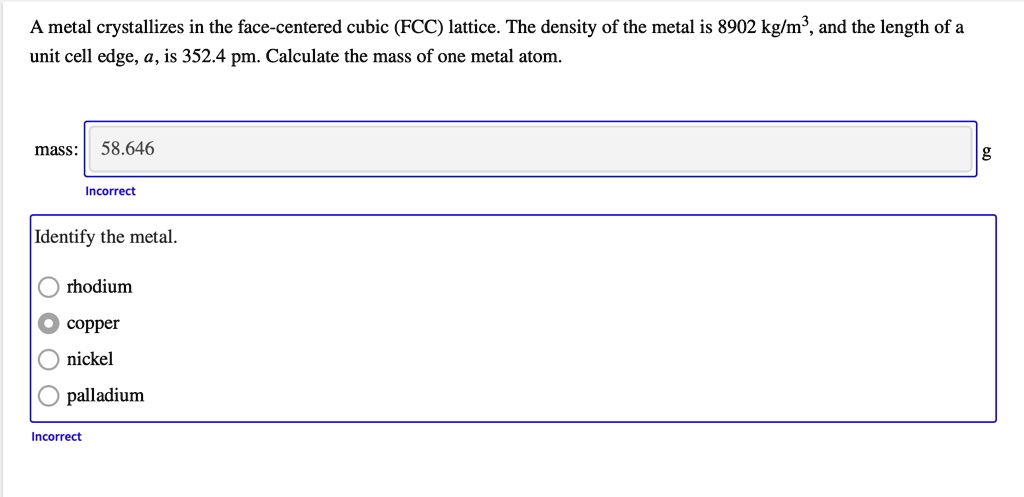
SOLVED: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is 8902 kglm , and the length of a unit cell edge, a,is 352.4 pm. Calculate the

SOLVED: Rhodium has a density of 12.41 g>cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.

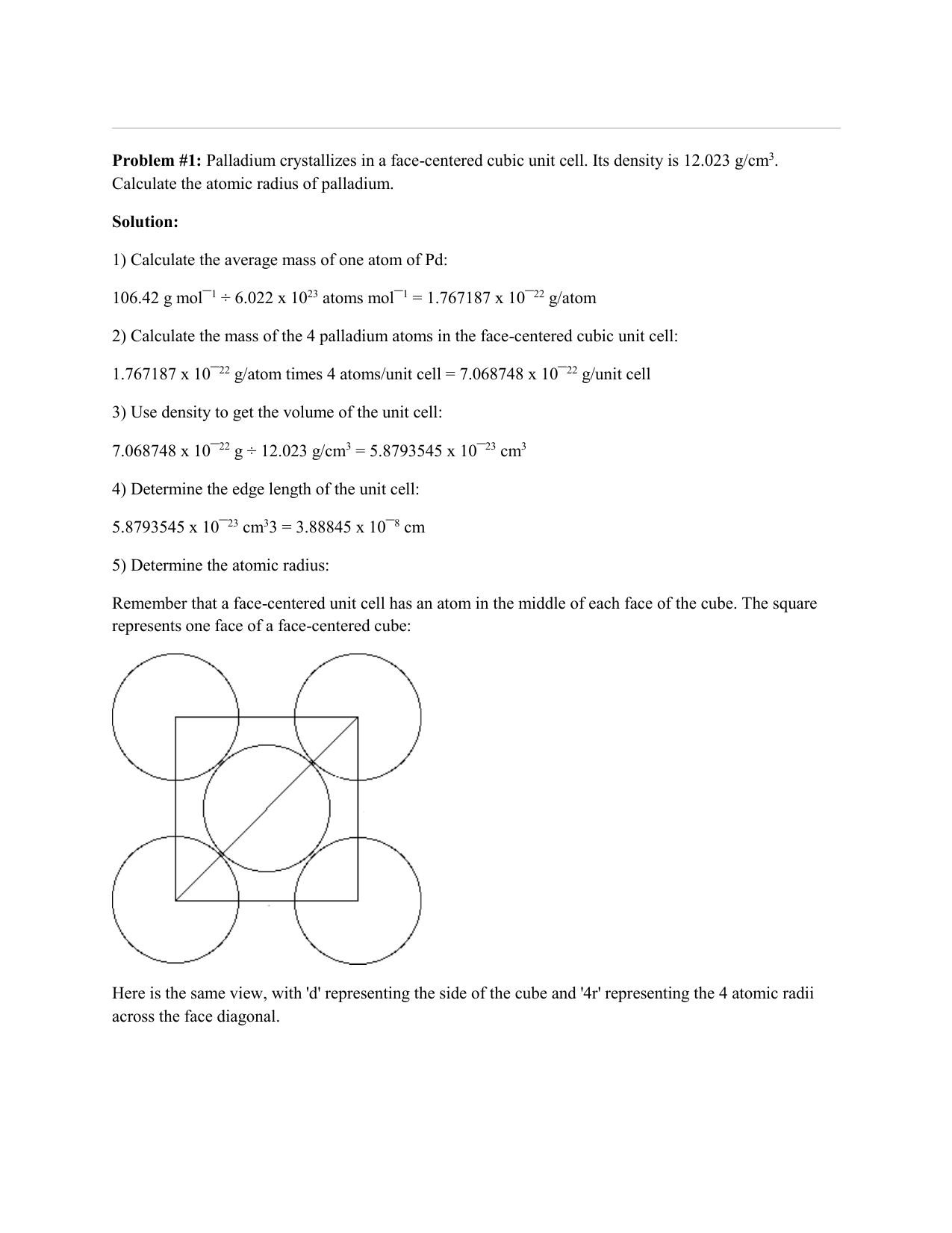
Problem.docx - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius | Course Hero

A Ketimide-Stabilized Palladium Nanocluster with a Hexagonal Aromatic Pd7 Core | Inorganic Chemistry